The highest level of scientific evidence supports cost-effectiveness of homeopathy – a finding that is unchanged by a recent paper by Leemhuis & Seifert
31 mayo 2024A systematic review – considered the highest level of scientific evidence – was published in January 2024, providing an overview of cost-effectiveness studies of homeopathy1. In all 21 studies included in this review, homeopathy showed similar or better clinical effectiveness compared to the control groups, with a clear positive trend for cost-effectiveness:
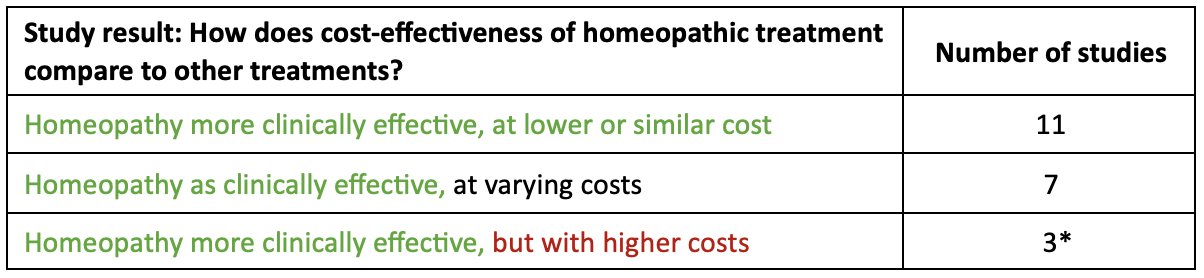
*2 of these studies were shown to be cost-effective through incremental cost effectiveness analysis.
The quality of the studies was assessed using the CHEC list, specifically designed for use with economic evaluations. CHEC scores ranged from 2 to 16 (maximum score 19), with a statistically significant increase in study quality over the years (see HRI Cost-effectiveness FAQ for further details).
Lead author, Prof Thomas Ostermann of Universitat Witten/Herdecke1 concluded that “although the quality of research on homeopathy’s cost-effectiveness has improved over time, and some high-quality studies show that it can be a cost-effective option, there are still many poorly conducted studies which make it difficult to offer a definitive statement.”
We therefore read with interest a new paper by Leemhuis & Seifert2 published in March 2024, titled, “Prescriptions of homeopathic remedies at the expense of the German statutory health insurance from 1985 to 2021: scientific, legal and pharmacoeconomic analysis”. However, regrettably, this study fails to meet basic scientific standards for a meaningful economic evaluation and, as such, adds nothing of value to the evidence base on this topic.
The quality of the study cannot be formally assessed using the CHEC-list tool, as it does not sufficiently resemble a true clinical cost effectiveness study3 (only 12 of the 19 checklist items could be assessed, with the study scoring 4/12 for those items), but the fact that Leemhuis & Seifert is a poorly conducted study is clear.
An over-simplified approach was used to track the cost per daily dose of 16 homeopathic medicines prescribed by doctors in outpatient clinics and paid for through the German statutory health insurance (SHI) from 1985 to 2021. Using this crude data they concluded that homeopathy costs “have continued to rise over the years”. But the data were not adjusted for inflation or compared to changes in conventional medicine costs over the same time period.
Leemhuis & Seifert also concluded that homeopathy was “on average significantly more expensive” than 9 selected alternative conventional medicines. However, this claim is based on a subset of data monitoring homeopathic medicines during a single year only (2021) and is further weakened by the following issues:
- The authors state that they found a “significant” difference, yet no statistical analyses were performed
- The conventional medicines selected as comparators for each homeopathic medicine were poorly chosen e.g. homeopathic nasal decongestant spray Euphorbuim comp. was compared to ibuprofen and paracetamol tablets, instead of conventional nasal decongestant spray xylometazoline, used elsewhere in the study.
- The authors failed to compare the clinical effectiveness of the ‘matched’ treatments – an essential step when considering the true value of any treatment.
When attempting to apply this paper’s findings to real world decision-making, further concerns arise. The homeopathic medicines tracked were prescribed for patients <12 years, yet no consideration was given to the particular issues associated with treating such young patients. For the treatment of intestinal colic, Leemhuis & Seifert2 compared a homeopathic suppository manufactured for babies, to the anti-spasmodic drug butylscopolamine, claiming it to be a ‘rational’ conventional alternative, even though this drug is contraindicated in children under the age of 6 years.
Finally, the authors fail to mention that the overall cost of homeopathy on the SHI (in 2022) was only 0.01% of total drug expenditure4 – a figure which would have put their findings in appropriate context.
Given the extent of these shortcomings, and their obvious impact on the reliability of the study’s conclusions, a Letter to the Editor is warranted to alert the journal to the poor quality of the paper. However, as the senior author of the paper, Prof Seifert, is himself the Editor-in-Chief of the journal which published his study, it is unlikely that this would prove worthwhile.
In conclusion, the paper by Leemhuis and Seifert2 is misleading and of inadequate quality due to a collection of weaknesses, including use of an unknown, non-replicable method; failure to substantiate their key findings in a way that is compliant with EBM and selective citation.5
The study therefore provides no useful insights into the likely cost and clinical effectiveness of homeopathic medicines, as prescribed by doctors in the German public health system. The systematic review by Ostermann et al.1 remains the most robust study on cost effectiveness to date, and the positive trends it identifies, based on studies from seven European countries6, supports the likely cost-effectiveness of homeopathy.
References
- Ostermann T, Burkart J, de Jaegere S, Raak C, Simoens S. Overview and quality assessment of health economic evaluations for homeopathic therapy: an updated systematic review. Expert Rev Pharmacoecon Outcomes Res 2024;24:117-142 | PubMed
- Leemhuis H and Seifert R. Prescriptions of homeopathic remedies at the expense of the German statutory health insurance from 1985 to 2021: scientific, legal and pharmacoeconomic analysis. Naunyn Schmiedeberg’s Arch Pharmacol 2024: online ahead of print | PubMed
- Evers S, et al. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care, 2005;21(2):240-5 | PubMed
- BPI. Pharma-Daten 2022. Page 78. Available from: https://www.fakom.de/missbrauch-von-wissenschaft-fuer-politische-desinformation/#:~:text=BPI%20Pharma%2DDaten%202022%2C%20S.78
- Mosley AJ. Pharmacoeconomic Study of Homeopathic Medicines: A Critical Appraisal of Methods and Conclusions Shows Serious Cause for Concern. Homeopathy 2024; 113(04): 274-278 | PubMed
- Source of studies included in Ostermann et al. 2024 review: Germany (n=8), UK (n=4), Italy (n=3), France (n=2), Switzerland (n=2), Belgium (n=1) and Netherlands (n=1).












