Clinical trials overview
Systematic reviews of homeopathy clinical trials
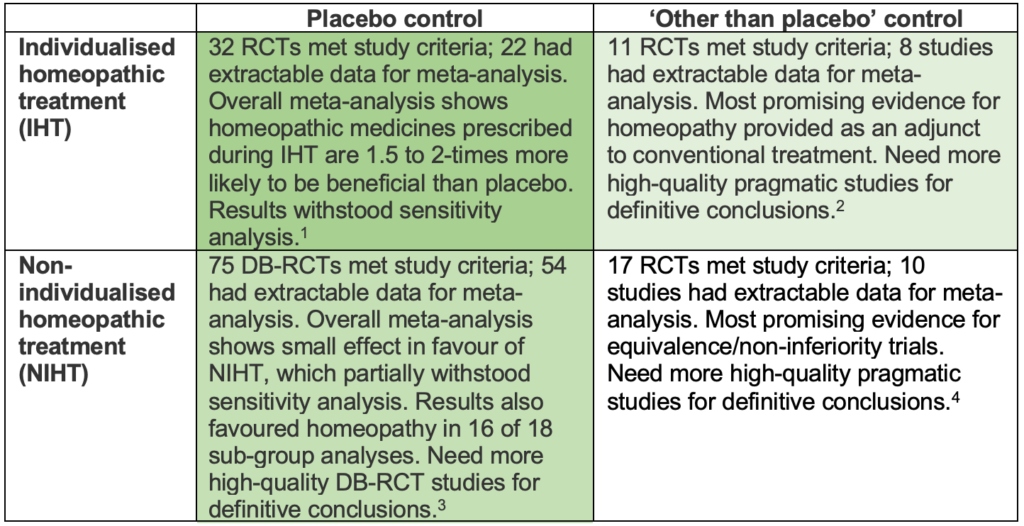
The most robust data on efficacy of homeopathy comes from a 2014 meta-analysis of placebo-controlled double-blind randomised controlled trials which found that homeopathic medicines, when prescribed during individualised treatment, are 1.5- to 2.0-times more likely to have a beneficial effect than placebo.1
This study is one of four comprehensive systematic reviews conducted by Dr Robert Mathie within a ten-year programme of work. These reviews made considerable strides in clarifying the evidence base by separately analysing groups of trials according to both the type of treatment (individualised or non-individualised homeopathy) and comparators (whether the control group was given placebo or something ‘other than placebo’).
Individualised homeopathic treatment is a tailored prescription provided after consultation with a homeopathic practitioner (somewhat similar to a visit to your GP/medical specialist). This is very different from non-individualised homeopathy in which the same homeopathic medicine is used by all patients, usually an over-the-counter medicine bought at a pharmacy containing multiple homeopathic ingredients.
The next step for homeopathy researchers will be to conduct more clinical trials on specific medical conditions such that there is sufficient data to reach definitive conclusions about how effective homeopathy is for individual medical conditions.
When taken collectively, Dr Mathie’s programme of work includes some important positive findings for homeopathy.
Summary of Dr Mathie’s Systematic Review Programme
Individualised homeopathic treatment
Taken together, the two systematic reviews on individualised homeopathic treatment1,2 provide the most rigorous and up-to-date assessment of the key question, ‘Does homeopathy work when prescribed as ‘usual care’ by a trained practitioner?’ The answer is clearly ‘yes’ – the data shows individualised homeopathic medicines to have an effect beyond placebo.
Non-individualised homeopathic treatment
The evidence for non-individualised homeopathy is promising, but less clear. Analysis of 54 placebo-controlled trials showed a small beneficial effect beyond placebo (SMD -0.33; 95% CI -0.44 to -0.21)3 that partially withstood sensitivity analysis and remained positive in 16 of 18 sub-analyses.
Overview of global systematic reviews with meta-analysis
An overview of systematic reviews was published in October 20235. This study included all reviews with meta-analysis comparing homeopathy against placebo for all medical conditions. The body of evidence assessed comprised 4 historical reviews published from 1997 to 20056,7,8,9 plus the two reviews on placebo-controlled trials by Mathie et al. (2014 and 2017).
Using conventional methods for synthesising evidence from multiple reviews, Hamre et al. concluded that the confidence in the cumulative evidence for a significant positive effect for homeopathy beyond placebo is High for individualised homeopathy, Moderate for non-individualised homeopathy and Moderate when both types of homeopathic treatment are analysed together.
These findings lend further credibility to the over-arching positive trend seen in reviews of homeopathy, or as the authors’ state,
“In contrast to frequent claims, the available meta-analyses of
homoeopathy […] show significant positive effects beyond placebo5.”
– Dr Harald Hamre, Institute for Applied Epistemology and Medical Methodology at Witten/Herdecke, Germany
Systematic reviews of clinical trials without meta-analysis
The Australian NHMRC Homeopathy Review10 published in 2015 includes clinical trials up to 2010 (captured within SRs published up to 3 January 2013) and separates the data by medical condition. The study concluded that there was ‘no reliable evidence’ of effectiveness of homeopathy. This study attracted criticism for its unprecedented and unscientific methodology e.g. for a study to be ‘reliable’ it had to have a minimum of 150 participants and a quality rating of 5/5 on the Jadad scale. This resulted in only 5 of 176 included studies being categorised as ‘reliable’. Following widespread media attention, the NHMRC Chief Executive Prof Anne Kelso made a public statement that, “Contrary to some claims, the review did not conclude that homeopathy was ineffective.”11 NHMRC was investigated by the Commonwealth Ombudsman to answer charges of scientific misconduct in relation to this report, but as no independent scientific expert could be engaged to assess the evidence, no verdict was reached on these charges.
Further research is needed to consolidate and expand the current clinical trial evidence base in homeopathy. However, these systematic reviews and meta-analyses have identified areas of most promise, helping researchers know where to focus their efforts in order to inform decision-makers and patients about the potential of homeopathic treatments.
- Mathie RT et al. Randomised placebo-controlled trials of individualised homeopathic treatment: systematic review and meta-analysis. Systematic Reviews, 2014; 3: 142 | Full text
- Mathie RT et al. Systematic Review and Meta-Analysis of Randomised, Other-than-Placebo Controlled, Trials of Individualised Homeopathic Treatment. Homeopathy, 2018; 107(4): 229-243 |Full text
- Mathie RT et al. Randomised, double-blind, placebo-controlled trials of non-individualised homeopathic treatment: systematic review and meta-analysis. Systematic Reviews, 2017; 6(1):63 | Full text
- Mathie RT et al.Systematic Review and Meta-Analysis of Randomised, Other-than-Placebo Controlled, Trials of Non-Individualised Homeopathic Treatment. Homeopathy, 2019; eFirst | Abstract
- Hamre, H.J., Glockmann, A., von Ammon, K. et al. Efficacy of homoeopathic treatment: Systematic review of meta-analyses of randomised placebo-controlled homoeopathy trials for any indication. Syst Rev 12, 191 (2023) | Full text
- Linde, K. et al. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials. Lancet, 1997; 350:834–843 | PubMed
- Linde K, Melchart D. Randomized controlled trials of individualized homeopathy: a state-of-the-art review. J Altern Complement Med. 1998;4(4):371–88 | Abstract
- Cucherat, M., Haugh, M. C., Gooch, M. & Boissel, J. P. Evidence of clinical efficacy of homeopathy. A meta-analysis of clinical trials. HMRAG. Homeopathic Medicines Research Advisory Group. Eur. J. Clin. Pharmacol., 2000; 56: 27–33 | PubMed
- Shang A, Huwiler-Muntener K, Nartey L, et al. Are the clinical effects of homeopathy placebo effects? Comparative study of placebo-controlled trials of homeopathy and allopathy. Lancet, 2005; 366: 726–732 | PubMed
- Australian National Health and Medical Research Council (NHMRC) 2015 Homeopathy Review | Link[
- CEO Statement | Release of an annotated version of the 2012 draft report The Effectiveness of Homeopathy: an overview review of secondary evidence | Link




